Abstract
Background: Bruton tyrosine kinase inhibitors (BTKis) are important tools to treat B-cell malignancies. However, duration of treatment may be limited by adverse events (AEs). Zanubrutinib (zanu) is a BTKi approved for mantle cell lymphoma (MCL) and is in development for other hematologic malignancies. Data from phase 3 head-to-head trials of zanu vs ibrutinib (ibr) in pts with Waldenström macroglobulinemia (WM) or chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) demonstrated that pts treated with zanu showed lower rates of AEs leading to discontinuation (Blood 2020;136(18):2038-50; EHA 2021 LB1900). Preliminary results from BGB-3111-215 (NCT04116437) show that zanu was well-tolerated in pts who discontinued ibr and/or acalabrutinib (acala) treatment due to AEs (EHA 2021 EP642). Here, we report updated results from the BGB-3111-215 study with a median follow-up of 9 months.
Methods: This study is an ongoing US, phase 2, multicenter, single-arm, open-label study. The safety and efficacy of zanu monotherapy (160 mg twice daily or 320 mg once daily) were evaluated in pts with B-cell malignancies who met criteria for continued treatment after having become intolerant to prior BTKi therapy. Pts were divided into cohort 1 (pts who were intolerant to ibr only) and cohort 2 (pts who were intolerant to acala alone/and ibr). Pts with documented progressive disease (PD) on prior BTKi therapy were excluded. Efficacy and safety, including recurrence of intolerant AEs to the prior BTKi, were evaluated. AEs were assessed for severity, seriousness, and relation to zanu; as well as dose reductions, holds, or discontinuations. Response was assessed by investigators based on response criteria for their respective indications (Blood 2008;131:2745; J Clin Oncol 2012;30:2820; J Clin Oncol 2014;32:3059; Br J Haemtol 2013;160:171). Disease parameters from study entry were the baseline for response assessment. Mutational analysis was performed on pts who discontinued treatment, and data will be shared once available. To support clinical findings, kinase selectivity was assessed using Kinome profiling at 100X IC50 (against BTK) for zanu, ibr, acala and its major metabolite, M27 (Reaction Biology Corp).
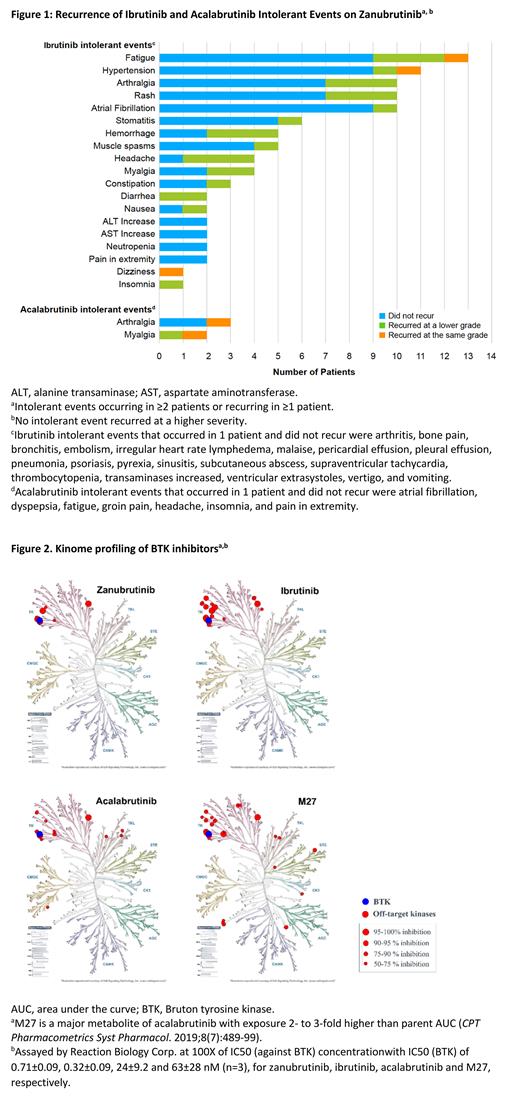
Results: As of 7 June 2021 (data cutoff), 57 pts (n=44 CLL/SLL; n=9 WM; n=2 MCL; n=2 marginal zone lymphoma [MZL]) were enrolled in cohort 1, and 7 pts were enrolled in cohort 2 (n=4 CLL; n=1 WM; n=1 MCL; n=1 MZL). All received ≥1 dose of zanu and were analyzed for safety. The median age was 71 years (range, 49-91) in cohort 1 and 71 years (range, 65-76) in cohort 2; median duration of treatment was 8.7 months (range, 0.6-17.9) in cohort 1 and 8.2 months (range, 6.4-11.4) in cohort 2; median number of prior regimens was 1 (range, 1-12) in cohort 1 and 3 (range, 2-5) in cohort 2. Within cohort 2, 5 pts were intolerant to both ibr and acala. Median number of intolerant events per pt for both cohorts 1 and 2 was 2 (range, 1-5). Overall, 73% of pts did not experience recurrence of their ibr or acala intolerant events and 79% of recurrent events recurred at a lower severity (Figure 1). At cutoff, 54 pts remained on treatment. Reasons for treatment discontinuation were AEs (n=4), PD (n=4), physician's decision (n=1), and consent withdrawal (n=1). Grade ≥3 AEs were reported in 18 pts (28%), and serious AEs occurred in 7 pts (11%). AEs requiring dose interruptions occurred in 17 pts (27%), and AEs leading to dose reduction occurred in 3 pts (5%). One death, due to COVID-19, was reported. Pts demonstrated maintained (41%) and improved (53%) response with zanu treatment from their reported best overall response on prior BTKis for a total disease control rate of 94% (including a 42% partial response rate in pts with CLL/SLL, 30% in pts with WM, and a 20% very good partial response rate in pts with WM). Zanu also demonstrated good selectivity by kinase profiling. It showed >50% inhibition on 7/370 kinases, while ibr, acala, and M27 had more off-target binding (17, 15 and 23 kinases, respectively) at their respective 100X IC50 (BTK) concentrations (Figure 2).
Conclusion: In pts with B-cell malignancies intolerant to ibr and/or acala, zanu treatment resulted in continued disease control or improved response. Zanu was well-tolerated, and most AEs that led to discontinuation of previous BTKi therapy did not recur or recurred at a lower grade. In support of clinical findings, differentiation between BTKi selectivity profiles favor zanu over ibr and acala.
Shadman: Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb, Morphosys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, and Atara Biotherapeutics, Adaptimmune: Consultancy; Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, Abbvie, TG Therapeutics, Beigene, AstraZeneca, Sunesis, Atara Biotherapeutics, GenMab: Research Funding; Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb, Morphosys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, and Atara Biotherapeutics, Adaptimmune: Membership on an entity's Board of Directors or advisory committees. Flinn: Nurix Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Seagen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; MorphoSys: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Forty Seven: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Calithera Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Verastem: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Curis: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Takeda: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; IGM Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; AbbVie: Consultancy, Other: All Consultancy and Research Funding payments made to Sarah Cannon Research Institute, Research Funding; Portola Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Trillium Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Rhizen Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Incyte: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Acerta Pharma: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Agios: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Kite, a Gilead Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Gilead Sciences: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Karyopharm Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Forma Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Genentech: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; ArQule: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Triphase Research & Development Corp.: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Roche: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Pfizer: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Teva: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Infinity Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Unum Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Celgene: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Constellation Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Juno Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; AstraZeneca: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Iksuda Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Loxo: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Merck: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Novartis: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Great Point Partners: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; BeiGene: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Janssen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; TG Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Century Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Hutchison MediPharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Vincerx Pharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Sarah Cannon Research Institute: Current Employment; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Seagen: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Unum Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute, Research Funding; Johnson & Johnson: Current holder of individual stocks in a privately-held company; Seattle Genetics: Research Funding. Levy: Epizyme: Consultancy, Other: Promotional speaker; Amgen Inc.: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Gilead Sciences, Inc.: Consultancy, Honoraria, Speakers Bureau; GSK: Consultancy, Other: Promotional speaker; Morphosys: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Beigene: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Novartis: Consultancy, Other: Promotional speaker; Dova: Consultancy, Other: Promotional speaker; TG Therapeutics: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Janssen Pharmaceuticals: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau. Burke: SeaGen: Consultancy, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; MorphoSys: Consultancy; Bristol Myers Squibb: Consultancy; AstraZeneca: Consultancy; Epizyme: Consultancy; Verastem: Consultancy; Kura: Consultancy; Kymera: Consultancy; AbbVie: Consultancy; Adaptive Biotechnologies: Consultancy; Roche/Genentech: Consultancy; X4 Pharmaceuticals: Consultancy. Cultrera: Beigene: Research Funding. Yimer: Astrazeneca: Speakers Bureau; Karyopharm: Current equity holder in publicly-traded company, Speakers Bureau; Janssen: Speakers Bureau; Beigene: Speakers Bureau; GSK: Speakers Bureau; Sanofi: Speakers Bureau; Amgen: Speakers Bureau; Pharmacyclics: Speakers Bureau; Texas Oncology: Current Employment. Chaudhry: Medical Oncology Associates, PS (dba Summit Cancer Centers): Current Employment; Novartis, Immunomedics: Current holder of individual stocks in a privately-held company. Gandhi: TG Therapeutics: Honoraria; Karyopharm Therapeutics: Honoraria; GlaxoSmithKline: Honoraria. Kingsley: Comprehensive Cancer Centers of Nevada: Current Employment. Tumula: Texas Oncology: Current Employment. Manda: Morphosys: Honoraria; Genmab: Current equity holder in publicly-traded company. Chen: BeiGene: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months. Cohen: BeiGene: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, Expenses. By: BeiGene, Ltd: Current Employment. Xu: Beigene: Current Employment; AstraZeneca: Ended employment in the past 24 months. Liu: BeiGene Co., Ltd: Current Employment, Current equity holder in publicly-traded company. Sharman: TG Therapeutics: Consultancy; Centessa: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC, an AbbVie Company: Consultancy; BMS: Consultancy; AbbVie: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy; Lilly: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal